Aggregating probabilistic predictions of the safety, efficacy, and timing of a COVID-19 vaccine
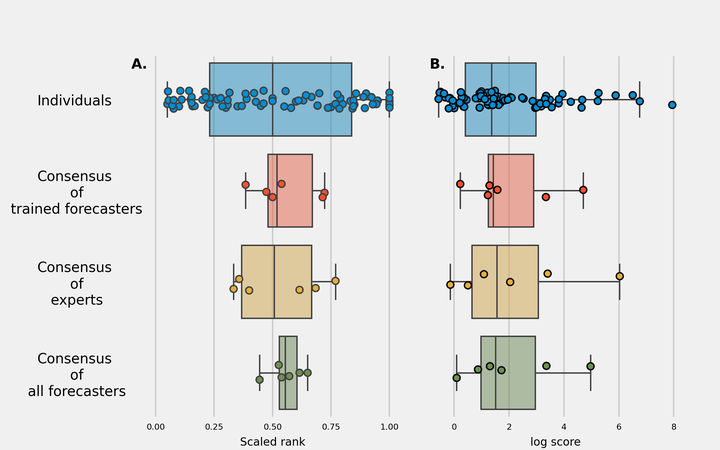
Safe, efficacious vaccines were developed to reduce the transmission of SARS-CoV-2 during the COVID-19 pandemic. But in the middle of 2020, vaccine effectiveness, safety, and the timeline for when a vaccine would be approved and distributed to the public was uncertain. To support public health decision making, we solicited trained forecasters and experts in vaccinology and infectious disease to provide monthly probabilistic predictions from July to September of 2020 of the efficacy, safety, timing, and delivery of a COVID-19 vaccine. We found, that despite sparse historical data, a consensus–a combination of human judgment probabilistic predictions–can quantify the uncertainty in clinical significance and timing of a potential vaccine. The consensus underestimated how fast a therapy would show a survival benefit and the high efficacy of approved COVID-19 vaccines. However, the consensus did make an accurate prediction for when a vaccine would be approved by the FDA. Compared to individual forecasters, the consensus was consistently above the 50th percentile of the most accurate forecasts. A consensus is a fast and versatile method to build probabilistic predictions of a developing vaccine that is robust to poor individual predictions. Though experts and trained forecasters did underestimate the speed of development and the high efficacy of a SARS-CoV-2 vaccine, consensus predictions can improve situational awareness for public health officials and for the public make clearer the risks, rewards, and timing of a vaccine.